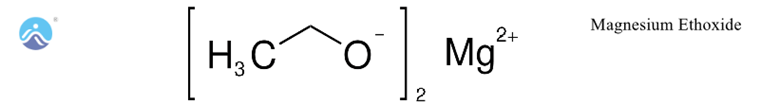Uses of Magnesium Ethoxide
2024-09-20Magnesium Ethoxide has the following various uses:
I. In the Field of Organic Synthesis
1. Introduction of Functional Groups
- In organic synthesis reactions, magnesium ethoxide can be used to introduce the ethoxy (-OC₂H₅) functional group into organic molecules. For example, when reacting with halogenated hydrocarbons, the ethoxy group in magnesium ethoxide can replace the halogen atom, generating organic compounds containing the ethoxy group. This reaction is of great significance in constructing complex organic molecular structures, especially in drug synthesis and the manufacture of fine chemicals.
- For some compounds containing a carbonyl group (C = O), such as aldehydes and ketones, magnesium ethoxide can undergo an addition reaction. During the reaction, the ethoxy group is added to the carbonyl carbon, and after a series of reaction steps, the molecular structure can be modified, thus synthesizing organic compounds with specific structures and functions.
2. Drug Synthesis
- In the pharmaceutical industry, magnesium ethoxide is widely used. The synthesis of many drug molecules requires specific reaction conditions and reagents to construct precise chemical structures. Magnesium ethoxide can participate in the synthesis processes of some antibiotics, antiviral drugs, and cardiovascular drugs, etc. For example, in the synthesis of some β - lactam antibiotics, magnesium ethoxide may act as a key reaction reagent, building the core structure of the drug molecule or introducing necessary functional groups through reaction with substrate molecules.
3. Fragrance Synthesis
- In fragrance chemistry, magnesium ethoxide is helpful for synthesizing compounds with unique scents. By reacting with fragrance precursor molecules, it can change the structure and properties of the molecules, thereby producing new fragrant substances. For example, in the synthesis of some ester - type fragrances, magnesium ethoxide can react with the corresponding carboxylic acids and alcohols, generating ester - type compounds with charming aromas through specific reaction mechanisms. These compounds can be used in the fields of perfumes, cosmetics, and food fragrances.
II. In Terms of Catalytic Effects
1. Catalysis in Polymerization Reactions
- Magnesium ethoxide can be used as a catalyst in some polymerization reactions. In the synthesis process of some polyesters, it can promote the polycondensation reaction between monomer molecules. Magnesium ethoxide interacts with the functional groups in the monomer molecules, reducing the activation energy of the reaction, making the reaction easier to carry out, thereby increasing the reaction rate and affecting the selectivity of the polymerization reaction. This catalytic effect helps to control the molecular weight, molecular structure, and physicochemical properties of the polyester, making it suitable for different application fields, such as plastics and fiber manufacturing.
2. Catalysis in Other Organic Reactions
- In some organic reactions, magnesium ethoxide can catalyze the transesterification reaction between alcohols and esters. This reaction is very useful for adjusting the structure and properties of ester - type compounds. Through the transesterification reaction, one type of ester can be converted into another type of ester, thereby changing its physicochemical properties, such as solubility and volatility, to meet different industrial requirements. Magnesium ethoxide activates the reaction substrate molecules during this process, enabling the transesterification reaction to be carried out under relatively mild conditions.