Sodium Citrate
Hosea Chem® has been supplying Sodium Citrate (CAS 68-04-2) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Sodium Citrate
Chemical Name:Sodium Citrate;CAS 68-04-2
EINECS No.: 200-675-3
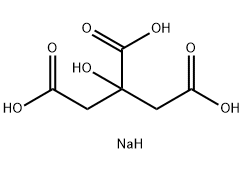
Chemical Formula: C6H5Na3O7
Molecular Weight: 258.07
Melting point: 300°C
Boiling point: 309.6℃
Flash point: 155.2°C
Density at 20°C: 1.008 g/mL
Molecular Structure:
Description
Sodium citrate is the sodium salt of citric acid. It is white, crystalline powder or white, granular crystals, slightly deliquescent in moist air, freely soluble in water, practically insoluble in alcohol. Like citric acid, it has a sour taste. From the medical point of view, it is used as alkalinizing agent. It works by neutralizing excess acid in the blood and urine. It has been indicated for the treatment of metabolic acidosis.
Sodium Citrate Standard
Appearance: White to off-white crystalline powder
Content %≥: 99.0
Density at 20°C: 1.008 g/mL
Vapor pressure (25℃): 5.73E-05mmHg
Application
Sodium Citrate is a buffer and sequestrant obtained from citric acid as sodium citrate anhydrous and as sodium citrate dihydrate or sodium citrate hydrous. the crystalline products are prepared by direct crystallization from aqueous solutions. sodium citrate anhy- drous has a solubility in water of 57 g in 100 ml at 25°c, while sodium citrate dihydrate has a solubility of 65 g in 100 ml at 25°c. it is used as a buffer in carbonated beverages and to control ph in preserves. it improves the whipping properties in cream and pre- vents feathering of cream and nondairy coffee whiteners. it pro- vides emulsification and solubilizes protein in processed cheese. it prevents precipitation of solids during storage in evaporated milk. in dry soups, it improves rehydration which reduces the cooking time. it functions as a sequestrant in puddings. it functions as a complexing agent for iron, calcium, magnesium, and aluminum. typical usage levels range from 0.10 to 0.25%. it is also termed trisodium citrate.
Sodium citrate is chiefly used as a food additive E331, usually for flavor or as a preservative. Sodium citrate is employed as a flavoring agent in certain varieties of club soda. Sodium citrate is common as an ingredient in Bratwurst, and is also used in commercial ready to drink beverages and drink mixes, contributing a tart flavour. As a conjugate base of a weak acid, citrate can perform as a buffering agent or acidity regulator, resisting changes in pH. Sodium citrate is used to control acidity in some substances, such as gelatin desserts. It can be found in the mini milk containers used with coffee machines. The compound is the product of antacids, such as Alka- Seltzer, when they are dissolved in water. Sodium citrate is used to relieve discomfort in urinary tract infections, such as cystitis, to reduce the acidosis seen in distal renal tubular acidosis, and can also be used as an osmotic laxative.
Storge & Handling
Stored in a cool, dry, and well-ventilated area, away from direct sunlight, heat sources, and incompatible materials. It is important to keep the container tightly closed when not in use.
Packing
25KG/Bag