Dimethyl Sulfoxide (DMSO)
Hosea Chem® has been supplying Dimethyl Sulfoxide (CAS 67-68-5) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Dimethyl Sulfoxide (DMSO)
Chemical Name:Dimethyl Sulfoxide;DMSO;CAS 67-68-5
EINECS No.: 200-664-3
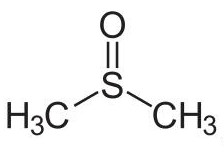
Chemical Formula: C2H6OS
Molecular Weight: 78.13
Melting point: 18.4°C
Boiling point: 189℃
Flash point: 192°F
Density at 20°C: 1.100 g/mL
Molecular Structure:
Description
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high melting point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after contact with the skin. DMSO is a 2-carbon sulfoxide in which the sulfur atom has two methyl substituents. It has a role as polar aprotic solvent, radical scavenger, non-narcotic analgesic, antidote, MRI contrast agent, Escherichia coli metabolite and alkylating agent. DMSO appears as a clear liquid, essentially odorless. Vapors are heavier than air.
Dimethyl Sulfoxide Standard
Appearance: Colourless clear liquid
Content %≥: 99.0
Density at 20°C: 1.100 g/mL
Acidity (pKa 25℃): 35
Vapor pressure (20℃): 0.42 mmHg
Refractive index n20/D: 1.479
Explosive limit %(v): 1.8-63.0
Application
1. DMSO is frequently used as a solvent for chemical reactions involving salts, most notably Finkelstein reactions and other nucleophilic substitutions. It is also extensively used as an extractant in biochemistry and cell biology.
2. DMSO is used in polymerase chain reaction (PCR) to inhibit secondary structures in the DNA template or the DNA primers. It is added to the PCR mix before reacting, where it interferes with the self-complementarity of the DNA, minimizing interfering reactions. DMSO may also be used as a cryoprotectant, added to cell media to reduce ice formation and thereby prevent cell death during the freezing process.
3. In medicine, DMSO is predominantly used as a topical analgesic, a vehicle for topical application of pharmaceuticals, as an anti-inflammatory, and an antioxidant. Because DMSO increases the rate of absorption of some compounds through biological tissues, including skin, it is used in some transdermal drug delivery systems. Its effect may be enhanced with the addition of EDTA. It is frequently compounded with antifungal medications, enabling them to penetrate not just skin but also toenails and fingernails.
Storge & Handling
Store in a dry, cool and ventilated place. Away from fire, sunlight.
Packing
225KG/Drum