Chloroacetic acid production process and main uses
2022-05-091. Chloroacetic acid production process
At present, this method is the main method for producing chloroacetic acid in the world. Large-scale chloroacetic acid production enterprises in the United States, Japan, Germany, the Netherlands, Canada and other countries have adopted this method for production, and some domestic enterprises have also adopted this method for production.
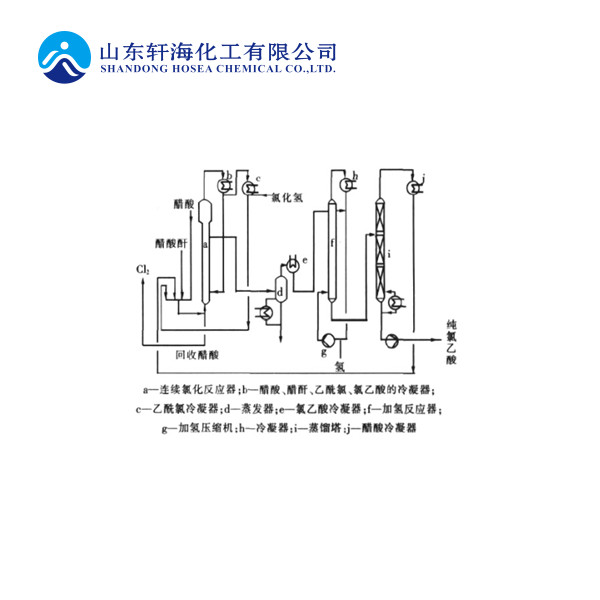
The process uses acetic acid and liquid chlorine as raw materials, acetic anhydride and (or) sulfuric acid as catalysts, through acetic acid chlorination, distillation, crystallization, separation, drying and other processes to obtain chloroacetic acid. The product obtained by this method has high quality, low consumption of raw materials, and less stringent requirements for raw material chlorine. It can be produced by liquid chlorine tail gas or gas chlorine. The disadvantage is that the reaction conversion rate is low, only about 45%, which increases steam consumption and power consumption.
Because the conversion rate of acetic acid is low, only 45%, that is, the chlorination reaction mixture contains a large amount of unreacted acetic acid. In order to reduce the crystallization load and effectively recover or utilize the unreacted acetic acid, methanol can be used to react to make its ester to produce methyl monochloroacetate and methyl dichloroacetate.
2. Main uses of chloroacetic acid
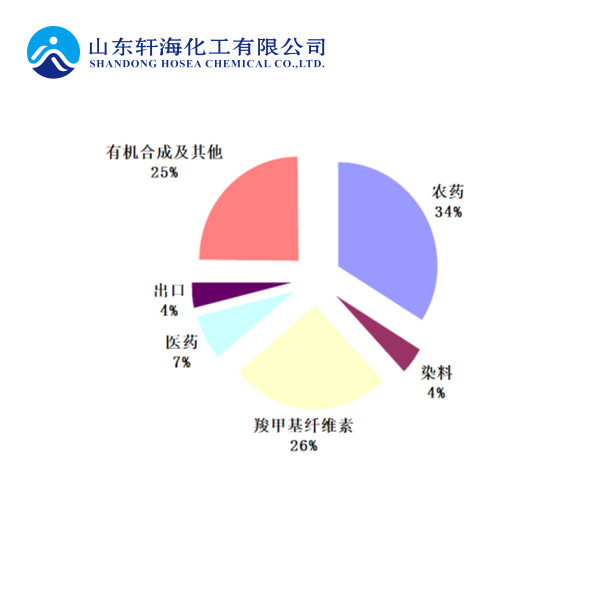
As an important fine chemical intermediate, chloroacetic acid is mainly used in the production of pesticides, sodium carboxymethyl cellulose, pharmaceuticals, dyes and the synthesis of other fine organic products.