Aluminium Chloride Anhydrous
Hosea Chem® has been supplying Aluminium Chloride Anhydrous (Cas 7446-70-0) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Aluminium Chloride Anhydrous
Cas No.: 7446-70-0
EINECS No.: 231-208-1
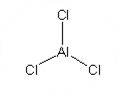
Chemical Formula: AlCl₃
Molecular Weight: 133.34
Melting point: 194°C
Boiling point: 178 °C
Density: 2.44 g/cm3
Molecular Structure:
Aluminium Chloride Anhydrous Standard
Appearance: Yellow or pale yellow, slightly gray granular or powder
Purity % ≥: 99.2
Fe ( FeCl3 )w/% ≤: 0.04
Water insolublesw/ % ≤ : 0.05
Heavy matel (Pb )w/ % ≤ : 0.006
Free aluminum w/ % ≤ : 0.010
Application
1. Anhydrous aluminum chloride is mainly used in Friedel-Crafts reaction, such as the preparation of anthraquinone from benzene and phosgene, which is used in the dyeing and finishing industry.
2. Anhydrous aluminum chloride is also often used to add aldehyde groups to benzene rings, such as the Gattermann-Koch reaction using carbon monoxide, hydrogen chloride, aluminum chloride and cuprous chloride as catalysts.
3. Anhydrous aluminum chloride has a wide range of applications in organic chemistry.
4. AlCl3 is also commonly used in hydrocarbon polymerization and isomerization reactions, important examples of which include the production of ethylbenzene in industry. Ethylbenzene can be used to further prepare styrene, polystyrene and dodecylbenzene used as a detergent.
5. AlCl3 can also be used as a catalyst for organic synthesis, such as petroleum cracking, synthetic dyes, synthetic rubber, synthetic detergents, medicines, fragrances, etc.
6. Anhydrous aluminum chloride is used to manufacture pesticides, organoaluminum compounds, catalysts for phthalocyanine organic pigments, and catalysts for the manufacture of ethylbenzene.
7. Anhydrous aluminum chloride is used in metal smelting and lubricating oil synthesis.
8. Food grade anhydrous aluminum chloride is used as a leavening agent, anti-discoloration agent for sake, and flocculant for pectin.
9. Anhydrous aluminum chloride is used as an analytical reagent, preservative, and mordant.
Storge & Handling
Keep containers tightly closed in a dry, cool and well-ventilated place.
Packing
25 Kgs/Bag.











