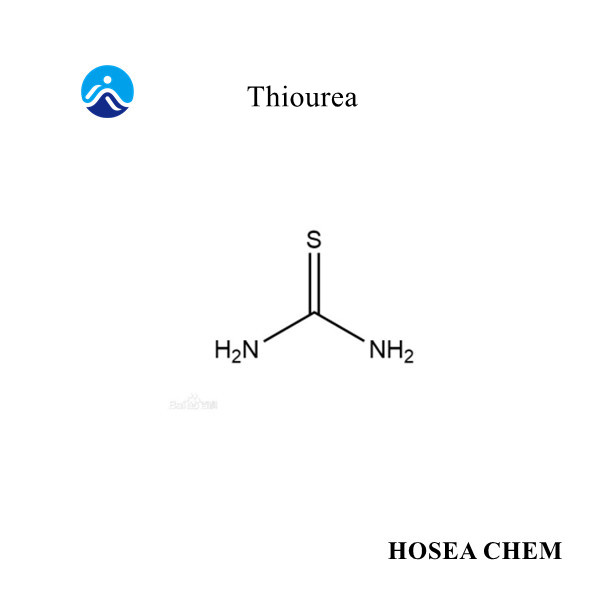
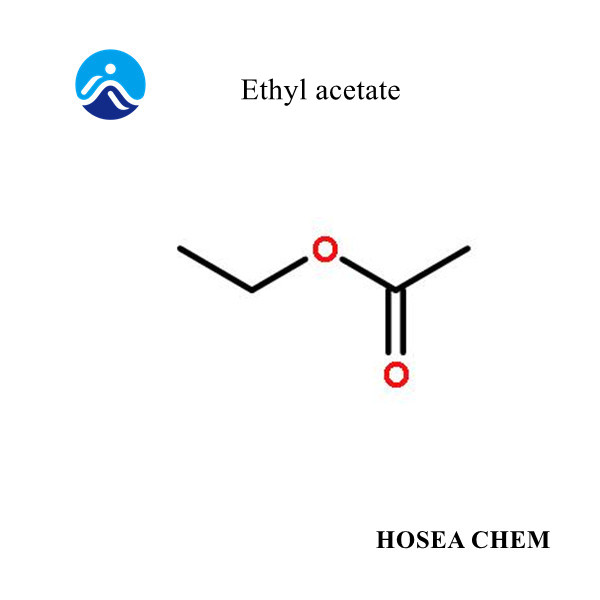
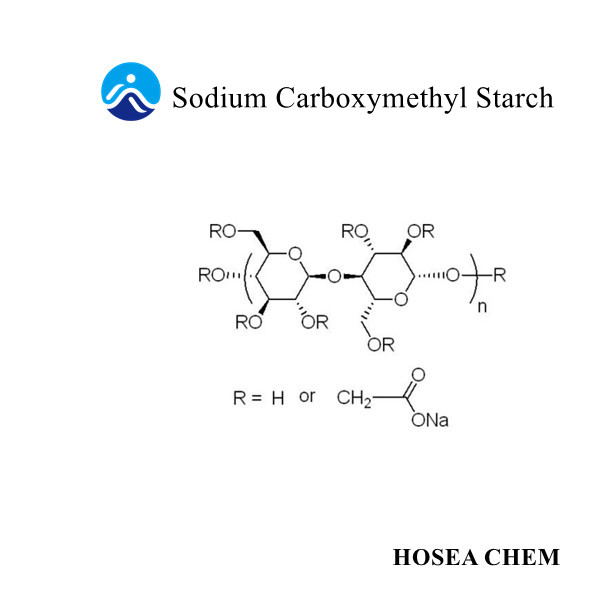
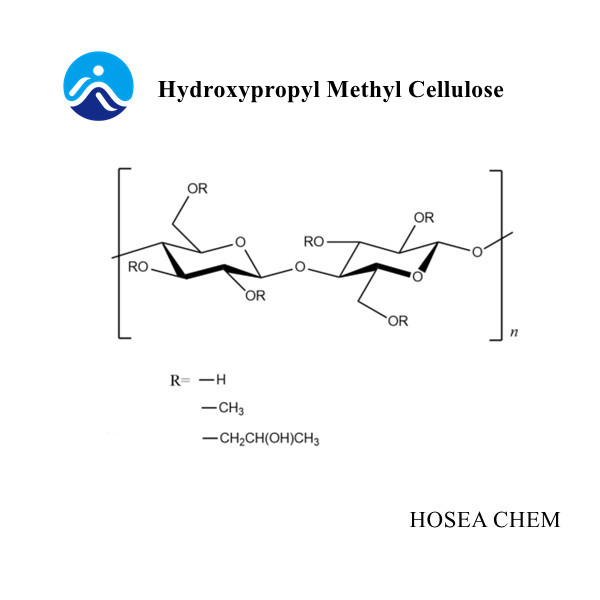
Introduction of sodium nitrite
2023-07-28Hosea Chem® Sodium nitrite (NaNO2) is an inorganic salt formed by the ionization and formation of nitrite ions and sodium.
Sodium nitrite is easy to deliquescence, soluble in water and liquid ammonia, its aqueous solution is alkaline, its pH is about 9, slightly soluble in organic solvents such as ethanol, methanol, ether.
It is a strong oxidizing agent and has reducing properties. It will gradually oxidize in the air, and the surface will become sodium nitrate, which can also be oxidized by oxidizing agents; when it encounters weak acids, it will decompose and release brown nitrogen dioxide gas; contacting with organic matter and reducing agents can cause explosion or combustion. , and release toxic and irritating nitrogen oxide gas; it can also be oxidized when encountering strong oxidants, especially ammonium salts, such as ammonium nitrate, ammonium persulfate, etc., can interact with each other at room temperature to generate high heat, causing combustibles to burn. Sodium nitrite reacts with oxygen to form sodium nitrate when exposed to air. If heated above 320°C, it will decompose and produce oxygen, nitrogen oxide and sodium oxide. It is easy to burn and explode when it comes into contact with organic matter.
Use:
Chromatographic analysis. Spot analysis is used to determine mercury, potassium and chlorate.
Diazotization reagent.
Nitrosating reagents.
soil analysis.
Serum bilirubin was measured in liver function tests.
Bleach for silk and linen.
Metal heat treatment agent.
Corrosion inhibitor for steel.
Antidote for cyanide poisoning.
Laboratory analysis reagents.
It is used as coloring agent, antimicrobial agent and preservative in meat product processing.
It is used in bleaching, electroplating and metal treatment, and is called industrial salt.
Storage precautions:
Sodium nitrite should be stored in a low-temperature, dry and ventilated warehouse. The doors and windows are tightly closed to prevent direct sunlight. It can be stored together with other nitrates except ammonium nitrate, but it should be stored separately from organic matter, combustibles and reducing agents, and isolated from fire sources.